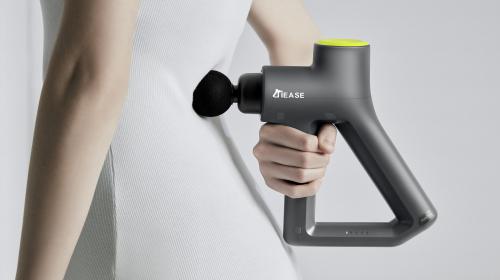
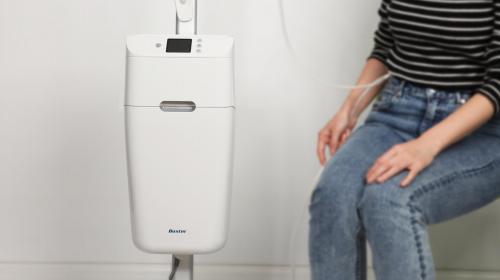
Medical Product Design and Development
At IDC, we specialise in medical product design and medical device development, delivering safe, effective, and commercially successful solutions for the healthcare sector. With offices in the UK and China, and our own in-house rapid prototyping and manufacturing facilities, we support clients worldwide to bring innovative medical products to market.
Some of the world's leading medical companies choose IDC because we deliver results. A recent analysis of client projects showed that 91% of our work went on to become successful products in the market – a record few in our industry could match. With over 45 years experience and hundreds of successful medical device developments, we guide clients through the entire process of getting a medical device designed, manufactured and approved in the most reliable and efficient way. Our London and Shanghai facilities are certified to ISO 13485 meaning we can support the approvals process for European CE (MDR), US FDA and China NMPA registration. We also offer medical device manufacturing services on behalf of our clients.
Speak to our expert team about your medical product design or device development project. Get in touch today.
Sign up for our top resources on medical product design and innovation
Our Medical Device Design Process
Our mission is simple. We innovate to create successful products that improve people's lives. We achieve this by bringing together some of the brightest and most highly motivated industrial designers and mechanical, electronics and software engineers, ensuring they are ready to solve your medical product design challenges using our proven, structured medical device development process.
Our four stage process covers everything from user research and human factors, through to design, engineering and prototyping to production, verification testing and market approval. From initial brief to production-ready design, our process is collaborative, transparent and driven by clinical insights. Every stage is geared towards regulatory readiness, manufacturability and innovation. Essential components of successful medical product design and device development.
Patents and IP are an increasingly important part of medical product design, with certain device areas having huge numbers of patents which need to be avoided. IDC has wide experience of avoiding competitor IP and securing patents for the innovative medical devices we create. In choosing to work with IDC you’ll be investing in a trustworthy partner who will ensure you not only get an innovative new product but you also get high quality, cost effective and reliable production delivered on time and on budget.
Thanks to our in-house facilities, we can rapidly prototype, test and refine medical devices, significantly reducing project risk and timelines. Our experience with Class I to III devices and global compliance frameworks makes us a trusted partner for both start-ups and major healthcare brands.
Start the conversation with our expert team today.
Key Services
Begin your product journey today
Our global team create market-leading products with aesthetics, safety and usability in equal measure. Get in touch with us today to begin your product journey.
What Our Clients Say About Us
[IDC led a professional, well coordinated and clear development programme. The overall completed design achieved our desired goals and the product remains one of the most innovative 'all in one' video laryngoscopes available.
]
[Our investment in IDC has been paid back many times over and we were delighted with their work. Not only was it a design for the future, but they produced it in a very timely and cost effective manner, and guided us through the process to ensure we avoided any pitfalls during manufacturing.
]
From early-stage concept to fully compliant manufacture, our specialists deliver world-class medical device development and product design. Start your project today.Partner with IDC for Expert Medical Innovation
Get in touch
For new business enquiries please contact our Studio Manager, Tiffany Hutchings.
contact@idc.uk.com | 01753 547 610
To find us, visit the Contact page.
Join us
We are always interested to hear from talented people who share our passion for product development.
Please visit our Careers page to learn more.