GECKO BIOMEDICAL
Surgical Adhesive Curing Device
Surgical precision
rapid development
IDC developed the Setalum light source for curing Gecko's innovative medical adhesive in vascular reconstructive surgery. Starting with a very basic product requirement, IDC overcame a range of user and technical challenges to deliver an approved product for clinical trials in just 12 months.
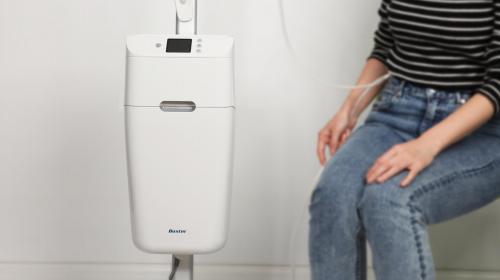
Working for Gecko Biomedical (now Tissium), IDC developed the Setalum Light - a first of its kind surgical adhesive curing device.
The device was designed to provide high quality curing of Tissium’s new Setalum adhesive which is used as an adjunct to suturing in vascular reconstructive surgery. After failing to find an off-the-shelf solution, IDC was briefed to develop a pen-sized, portable and rechargeable 405nm light source for curing its own surgical adhesive. The device needed to be entirely sterile for use in complex heart surgery.
IDC's team included mechanical and electronic engineers, industrial designers, and prototype builders, which meant it could take the product from initial requirement to clinical trials in just 12 months.
IDC spent considerable time researching and understanding user needs by observing surgery and interviewing clinicians. Clinicians also completed a questionnaire and tested block models which provided valuable information about user expectations and procedure.
Desk research gave a useful insight into the technology available and showed that there were no similar sterile light source products available. The team tested and evaluated all types of LED products to find the best LED light solutions.




One of the biggest challenges identified during clinical observation, was the need for a parallel light beam to give the correct light intensity even when the device was held at different heights. IDC’s design team developed a solution which used a collimated lens configuration combined with a custom prism to adjust the 405nm wavelength light beam angle, making it easier to position.
The other key issue of sterility was overcome by designing a disposable sterile sleeve to encase the device, along with a failsafe procedure amongst the surgical team to ensure sterility while applying the sterile sleeve to the device. As an additional safety feature, IDC included infrared sensors in the design to prevent the light source from being used when not placed inside the sterile sleeve.
Being a pen-sized device, the team was also challenged to fit the electronics into a small physical space. With a strategy for simplicity, the team used the least number of components possible to reduce risk and unnecessary cost.
Risk was also reduced by using programmable logic for the electronics, which ensured a clear verification process (compliant with ISO 60601). The device was powered by a rechargeable battery with a dual device recharging base.
The overall design was made simple and intuitive for optimum curing performance by including details such as a single ‘on’ button at the top to initiate 30 seconds of curing time, and simple LED charge indicators to make it clear if the device was ready for use.
The final system resulted in a multi classification device in the EU with the reusable electronic light source being Class I and the sterile disposable sleeve being Class IIa. The system is also approved by the US FDA.

Sign up to receive our top resources on product development and innovation.